Source: ECS Journal of Solid State Science and Technology article
Electrochemical Society highlights the top five most-read journal articles in each area during each quarter of the year. For Q1 of 2019, “Capacitance Stability in Polymer Tantalum Capacitors with PEDOT Counter Electrodes” by Dr. Yuri Freeman, et al., was the top read article in the Dielectric Science and Technology category.
by Y. Freeman 1, I. Luzinov 2, R. Burtovyy 2, P. Lessner 1, W. R. Harrell 3, S. Chinnam 3 and J. Qazi 1
1] KEMET Electronics Corporation, Simpsonville, South Carolina 29681, USA
2] Department of Materials Science and Engineering, Clemson University, Clemson, South Carolina 29634, USA
3] Holcombe Department of Electrical & Computer Engineering, Clemson University, Clemson, South Carolina 29634, USA
Abstract
Polymer Tantalum capacitors with PEDOT cathodes fabricated with an aqueous pre-polymerized PEDOT dispersion (slurry PEDOT) were investigated for stability with temperature. In particular, capacitance dependence on temperature was investigated in humidified and dry capacitors fabricated with both coarse and fine tantalum powders, variable thicknesses of the Ta2O5 dielectric, and in-situ vs. slurry PEDOT cathodes. Electrical measurements along with scanning electron microscopy (SEM) and secondary ion mass spectrometry (SIMS) were used to characterize the samples.
The capacitance of all samples was observed to increase with temperature; however, the extent and nature of this increase depends significantly on the nature of the polymer cathode and its interface with the dielectric. Humidified devices showed less capacitance variation above room temperature while dry devices showed less capacitance variation below room temperature for both coarse and fine powders. These results cannot be explained by the inherent variation of the dielectric constant with temperature.
A model was developed for the capacitance dependence on relative humidity and temperature based on surface area changes due to heating or cooling, complimented by changes in the dielectric constant, which is in good agreement with the experimental data. The results of this work demonstrate the critical impact of the dielectric-polymer interface on capacitance stability in these capacitors.
Introduction
Polymer Tantalum capacitors consist of porous tantalum anodes sintered in vacuum with tantalum powder, anodic oxide films of tantalum employed as a dielectric, and conductive polymer cathodes typically made of Poly(3,4-ethylenedioxythiophene) (PEDOT).1,2 Applications of Polymer Tantalum capacitors are rapidly increasing due to a combination of the high volumetric efficiency provided by the porous structure of the tantalum anode and the low equivalent series resistance (ESR) provided by the high conductivity of the PEDOT cathode. Recent improvements in the oxide dielectric and its interface with the polymer cathode have provided a radical increase in the ultimate breakdown voltage (BDV) and, thereby, the working voltage of Polymer Tantalum capacitors as well as a decrease in the dc leakage and failure rate in these capacitors.3,4 These improvements allowed for broad applications of Polymer Tantalum capacitors not only in commercial electronics, but also in high reliability special electronics such as military and space. In terms of working voltages, dc leakage, and failure rate, Polymer Tantalum capacitors are now comparable to Wet Tantalum capacitors, while their ac characteristics, such as ESR and capacitance stability with frequency, are much better than those of Wet Tantalum capacitors.
The important change to the technology that contributed to better performance and reliability of Polymer Tantalum capacitors relates to the process of applying a PEDOT cathode on the surface of the Ta2O5 dielectric. Traditionally it was an in-situ chemical reaction between the monomer and the oxidizer (in-situ PEDOT) followed by washing out by-products of the chemical reaction. The new process consists of dipping previously formed tantalum anodes in a water-based pre-polymerized PEDOT dispersion (slurry PEDOT) followed by drying in air at both room temperature and elevated temperature. The slurry PEDOT process does not have any by-products that contaminate the polymer cathode and its interface with the Ta2O5 dielectric.5,6 It is practically impossible to fully wash out by-products of the in-situ chemical reaction from the small pores in sintered and formed tantalum anodes, especially when they become filled with the polymer cathode, therefore the absence of by-products is a primary advantage of the slurry PEDOT process.
Despite these advantages and simplicity in mass manufacturing, the application of slurry PEDOT as an internal cathode in Polymer Tantalum capacitors is limited due to several issues. The major issue is that slurry PEDOT requires tantalum anodes with relatively large pores sintered with relatively coarse tantalum powder in order to allow the particles of the pre-polymerized PEDOT to impregnate the porous anodes. Even with these anodes, Polymer Tantalum capacitors with slurry PEDOT demonstrate higher ESR and anomalous transient currents.7 In addition, Polymer Tantalum capacitors with slurry PEDOT cathodes demonstrate stronger capacitance dependence on ambient conditions such as relative humidity and temperature in comparison to capacitors with in-situ PEDOT. The capacitance stability with humidity and operating temperature is important for many applications, especially in the case of non-hermetic surface mount Polymer capacitors. In this work, capacitance dependence on temperature was investigated in humidified and dry Polymer Tantalum capacitors fabricated with different tantalum powders, variable thicknesses of the Ta2O5 dielectric, and in-situ vs. slurry PEDOT cathodes. Scanning electron microscopy (SEM) and secondary ion mass spectrometry (SIMS) were combined with electrical measurements to characterize the samples. The results of this work demonstrate the critical impact of the dielectric-polymer interface on capacitance stability in these capacitors. A physical model was developed to explain the capacitance dependence on relative humidity and temperature, which is in good agreement with the experimental data.
Experimental
The fabrication of the tantalum anodes was performed with 12,000 μC/g and 50,000 μC/g tantalum powders pressed at 5.7 g/cc density into 1.32 g cylindrical pellets and sintered in vacuum at 1650°C for the coarser powder and 1350°C for the finer powder. The sintered tantalum anodes were then anodized in 0.1 wt% phosphoric acid. During the anodization the dc current density was approximately 1 mA/cm2, and the formation voltages varied from 9 V to 100 V. In-situ oxidative polymerization of PEDOT was performed by polymerization of 3,4-ethylenedioxythiophene in the presence of iron (III) toluene-sulfonate with a monomer/dopant ratio of 3:1.8 Pre-polymerized PEDOT was applied by dipping the sintered and anodized tantalum pellets into a water born dispersion of the nanoscale PEDOT particles, followed by drying in air at room temperature and then at 150°C.9–11 In some cases for comparison purposes, (3-aminopropyl)triethoxysilane was applied to the surface of the Ta2O5 dielectric prior to the PEDOT cathode in order to improve coverage of the dielectric with the polymer cathode inside porous anodes. After application of external carbon and silver layers, the capacitors were assembled into nickel plated brass cans with a glass insulator for positive external termination. Before sealing the hermetic cans, the capacitors were exposed to either 50% relative humidity at room temperature for 24 hours (Humid) or dry air at 125°C for 24 hours (Dry).
Capacitance measurements on the finished Polymer Tantalum capacitors were performed at 120 Hz with an Agilent E4980A precision LCR meter in the range of temperatures from −55°C to 125°C. The capacitance was normalized per unit of surface area (A) calculated from the specific charge of the powder (CV/g) and anode weight (P): [1]where the constant n = a/kko = 8.69 m2/FV; the volt coefficient, a = 2 × 10−9 m/V; the dielectric constant of Ta2O5, k = 26; and the vacuum permittivity, ko = 8.85*10−12 F/m.12 The variations in the capacitance readings between similar parts used in this paper were within +/-5%.
The coverage of the Ta2O5 dielectric with PEDOT cathode inside the porous tantalum anodes was analyzed using Scanning Electron Microscopy (SEM). A Hitachi SU3500 SEM operated at 6 kV was used with a 50 μm aperture. Tantalum anodes with different polymer impregnation treatments were fractured rather than cross-sectioned (to minimize sample preparation artifacts) for the SEM analysis. Backscattered electron imaging was performed at fixed distances from the surface to the center of the anodes to compare the internal polymer coverage. Multiple areas were examined in each case and representative images were captured.
Results and Discussion
The relative change in capacitance of Polymer Tantalum capacitors (Cpoly) with in-situ and slurry PEDOT in comparison to capacitance tested in a liquid cell (Cwet) after formation of the Ta2O5 dielectric is shown in Fig. 1 as a function of the formation voltage. The anodes in these capacitors were sintered with 50,000 μC/g tantalum powder, the capacitance was tested at room temperature, and capacitance change was defined as 100*(Cwet – Cpoly)/Cwet.
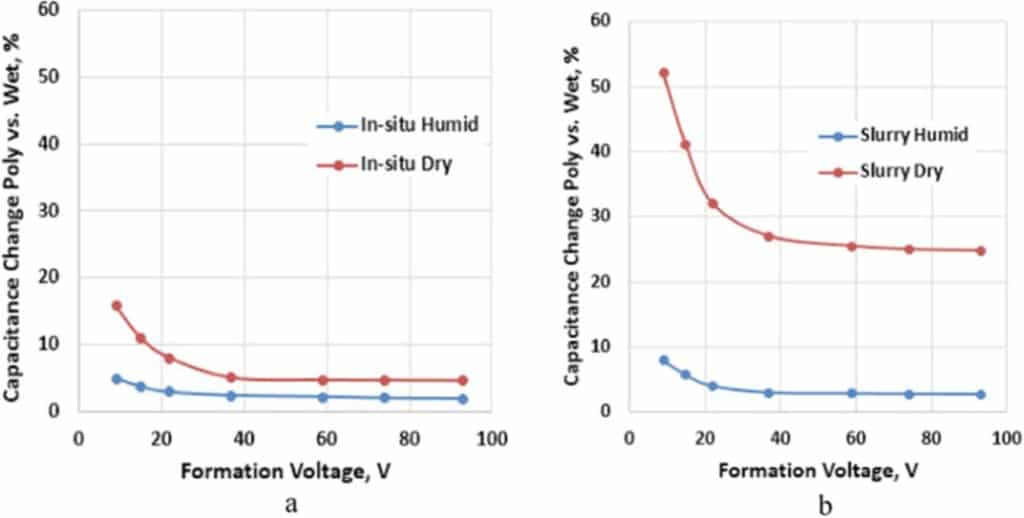
Figure 1.Relative change in capacitance for different formation voltages, Poly vs. Wet, of Polymer Tantalum capacitors with a 50,000 μC/g anode for (a) in-situ and (b) slurry PEDOT cathodes.
From Fig. 1 we see that the capacitance of humidified Polymer Tantalum capacitors with either in-situ or slurry PEDOT cathodes is similar to Wet capacitance after formation of the Ta2O5 dielectric. Except for very low formation voltages, there is no significant difference in capacitance between humidified and dry Polymer Tantalum capacitors with in-situ PEDOT cathodes; however, this difference is much larger in Polymer Tantalum capacitors with slurry PEDOT cathodes. The difference in capacitance between humidified and dry Polymer Tantalum capacitors can indicate how well the Ta2O5 dielectric is covered with PEDOT, and this coverage is much better for in-situ compared to slurry PEDOT in the case of tantalum anodes sintered with 50,000 μC/g tantalum powder.
The capacitance dependence on temperature for Polymer Tantalum capacitors with either 12,000 μC/g anodes or 50,000 μC/g anodes, a Ta2O5 dielectric formed at 44 V, and a slurry PEDOT cathode is shown in Fig. 2. According to Fig. 2, Polymer Tantalum capacitors with anodes sintered with coarser powder, (b), have a smaller difference in room temperature capacitance between humidified and dry capacitors in comparison to that of Polymer capacitors sintered with finer tantalum powder, (a). This difference can indicate better impregnation of the slurry PEDOT in tantalum anodes with larger pores in sintered anodes. The relative change in capacitance with temperature (ΔC) with respect to capacitance at room temperature (CRT) in these Polymer Tantalum capacitors is shown in Fig. 3, along with the relative change in dielectric constant (Δk) of the tantalum pentoxide with respect to the dielectric constant at room temperature (kRT).13 In this case ΔC = 100*[C(T) – CRT]/CRT and Δk = 100*[k(T) – kRT]/kRT.
Figure 2.Capacitance dependence on temperature for Polymer Tantalum capacitors with either (a) 12,000 μC/g or (b) 50,000 μC/g tantalum anodes, each with 44 V formation and slurry PEDOT cathodes.
Figure 3.Relative change in capacitance (ΔC) and dielectric constant (Δk) with respect to capacitance and dielectric constant respectively at room temperature, in humid and dry PHS Tantalum capacitors with anodes sintered with either (a) 12,000 μC/g or (b) 50,000 μC/g tantalum powders and slurry PEDOT cathodes.
From Fig. 3, we see that humidified Polymer Tantalum capacitors have a larger capacitance change with temperature below room temperature, while dry Polymer capacitors have a larger capacitance change with temperature above room temperature regardless of the type of tantalum powder in their anodes. Furthermore, the absolute change in capacitance with temperature is larger for dry capacitors above room temperature and humid capacitors below room temperature with anodes consisting of finer tantalum powder. In the given range of operating temperatures, the capacitance change with temperature exceeds 20% in capacitors with 50,000 μC/g tantalum anodes while it is below 20% in capacitors with 12,000 μC/g tantalum anodes. In addition, at temperatures below room the change in capacitance is small and practically identical for Dry Polymer Tantalum capacitors with both powders, while at temperatures above room, the change in capacitance is small and practically identical for Humid Polymer Tantalum capacitors with both powders.
Comparison of Fig. 2 and Fig. 3 indicates a correlation between the capacitance difference between humidified and dry samples at room temperature and the variation in capacitance with temperature with respect to capacitance measured at room temperature. In Polymer Tantalum capacitors with anodes sintered with coarser tantalum powder there is a smaller variation in the capacitance at room temperature between the humidified and dried samples, and there is also a smaller variation in capacitance with temperature with respect to the capacitance at room temperature. This correlation implies that better impregnation of tantalum anodes with slurry PEDOT due to the usage of tantalum anodes with more open pores or other technological means will improve capacitance stability with temperature in these devices.
The capacitance change in humidified Polymer Tantalum capacitors below room temperature is significantly higher than the change in the dielectric constant of the tantalum pentoxide. One of the possible causes for this phenomena can be the freezing of water in the porous anodes and, thereby, a change in the surface conductivity of the dielectric. To investigate this hypothesis, some of the Polymer Tantalum capacitors were impregnated with ethanol by drying of the capacitors in air followed by exposure to saturated ethanol vapor. The capacitance dependence on temperature in Polymer Tantalum capacitors with slurry PEDOT cathodes exposed to either water or ethanol vapor is presented in Fig. 4.
Figure 4.Capacitance dependence on temperature of Polymer Tantalum capacitors with slurry PEDOT exposed to either water or ethanol vapor.
According to Fig. 4 capacitance loss at low temperature is practically identical in Polymer Tantalum capacitors exposed to either water vapor or ethanol vapor; although, the freezing temperature of ethanol is −114°C which is much lower than the standard operating temperatures used in these experiments. These results show that the freezing of water inside porous anodes of Polymer Tantalum capacitors is not the dominant mechanism of the capacitance loss observed in these devices at low temperatures. From the data presented in Fig. 2 and Fig. 3, an alternative mechanism of capacitance change with temperature in Polymer Tantalum capacitors can be related to the coverage of the dielectric with the conductive polymer cathode. To improve this coverage, a Silane coupling agent was applied to the surface of the dielectric prior to slurry PEDOT by dipping of the formed tantalum anodes in a methanol based solution of the amine silane.14 The temperature dependence of capacitance in Polymer Tantalum capacitors with and without Silane is shown in Fig. 5 for 12,000 μC/g tantalum anodes.
Figure 5.The temperature dependence of capacitance in Polymer Tantalum capacitors with 12,000 μC/g tantalum anodes and either with or without Silane applied to the dielectric prior to the slurry PEDOT.
From Fig. 5 we see that applying amine Silane to the dielectric surface prior to slurry PEDOT reduced the difference in capacitance between humidified and dry Polymer Tantalum capacitors at room temperature, and improved the capacitance dependence on temperature in these capacitors over the entire range of operating temperatures. A high density of amine containing fragments, CH4N+, was detected by SIMS in finished Polymer Tantalum capacitors with amine Silane and slurry PEDOT, as shown in Fig. 6.
Figure 6.The peak of CH4N+ on the SIMS spectra of a Polymer Tantalum capacitor with amine Silane and slurry PEDOT.
Despite a complex chemical composition of the surface layer in a finished polymer tantalum capacitor (presence of PEDOT in addition to the Silane) a prominent spectral line at 30 m/z corresponding to the Silane layer is clearly visible in Fig. 6. The same characteristic line was observed for a layer of amino Silane deposited on a steal surface.15 The authors ascribed the line to the CH4N+ moiety that can originate from the NH2-CH2- end group in the Silane structure. Furthermore, the line was not observed in the case of other Silanes (3-glycidyloxypropyl)trimethoxysilane, 2-[methoxy(polyethyleneoxy)6-9propyl] trimethoxysilane, trimethylethoxysilane, and [3-(methacryloyloxy)propyl]trimethoxysilane) (spectra not shown) applied to the dielectric layer. The complexity of the surface layer composition does not allow for performing a more detailed analysis of the TOF SIMS spectrum. The spectrum is meant to serve the sole purpose of confirmation of the presence of the amino Silane layer.
SEM images in Figs. 7a and 7b show representative internal polymer coverage of fractured Tantalum capacitors with and without Silane treatment respectively. At the same distance from the surface of the Ta anodes, Silane treatment resulted in a thin but relatively homogeneous internal polymer coverage of the Ta-oxide dielectric particles, shown in Fig. 7a. Without the Silane treatment, the internal polymer is inhomogeneous in nature and thick in places, as seen in Fig. 7b. The difference in RT dry vs. humid capacitance of Ta anodes with no Silane treatment (Fig. 5) can be attributed to inhomogeneous Polymer coverage. Silane treated Ta anodes on the other hand, which show homogeneous polymer coverage, did not show a difference between RT dry vs. humid capacitance (Fig. 5). As a coupling agent the Silane treatment could provide better adhesion between the internal polymer and the dielectric over the measured temperature range and thus result in improved capacitance stability.
Figure 7.SEM images of fractured Polymer Tantalum capacitors with (a) and without (b) Silane treatment on Ta2O5 before Polymer slurry deposition.
Modeling and Analysis
The experimental results on the temperature dependence of capacitance after saturation with ethanol and water vapor clearly indicate that the freezing/melting phase transformation does not contribute significantly to the observed temperature effects. In fact, measurements on dry samples containing limited to no moisture reveal an increase in the capacitance beyond that expected from the normal dielectric constant effect at higher temperature, implying that freezing of water/alcohol plays no major role in the observed changes. In our opinion, the most plausible explanation for the observed phenomena can be determined through consideration of the effective surface area changes upon heating or cooling complimented by changes in the dielectric constant of Ta2O5.
The proposed model assumes that the PEDOT layer can shrink upon cooling and expand upon heating. As a result, the effective surface area contributing to the overall device capacitance decreases and increases, respectively. Therefore, the relative values of the capacitance change with temperature could be much lower/higher compared to such changes that are normally expected due to the variation with temperature of the dielectric constant of the oxide.
The model is based on several assumptions:
- General:
-
The change in the capacitance is a combination of changes in the oxide/PEDOT area of contact (effective surface area) and the dielectric constant of the oxide;
-
The surface coverage of the dielectric by the slurry PEDOT in a dry capacitor is only partial, leaving a certain amount of space for expansion of the polymer and therefore the effective surface area can increase. For example, the initial coverage of the 50,000 μC/g anode at 44 V is about 75% (Fig. 1b);
-
The coverage increases upon humidification (from 75% to about 97% for the same case as in Fig. 1b) because of swelling and expansion of the polymer. A new interface between the oxide and PEDOT is created.
-
- High temperature:
-
PEDOT can expand upon heating, increasing the surface coverage;
-
The expansion of the PEDOT for coverage close to 100% (humidified samples) is minimal (no room for expansion), and the changes in capacitance are caused primarily by variations in dielectric constant of the oxide layer only;
-
Dry PEDOT is capable of expanding upon heating to the level of the humidified polymer at room temperature;
-
- Low temperature:
-
PEDOT can shrink upon cooling, decreasing the surface coverage;
-
The adhesion between oxide and PEDOT at the interface created upon humidification, is much lower than that originally formed during PEDOT deposition. The newly formed interface is assumed to be in existence predominantly through the mechanical contact of the expanding PEDOT layer over the dielectric. Thus, the interface formed upon humidification can easily be delaminated from the surface during contraction of the material at low temperatures.
-
The schematic graphical representation of the changes at the oxide/PEDOT interface is illustrated in Fig. 8. Within the framework of the theoretical considerations just presented, it is possible to calculate the theoretical values of the capacitance changes based on the measured values of dry and humidified capacitors at room temperature. For simplicity, we consider only two practical extremes of temperatures, −55°C and 150°C. The model is presented below, in Equations 2–5,
where C is capacitance, Δɛ is the change in dielectric constant of the oxide relative to the value at 25°C, ΔCcontr is change in capacitance due to contraction of the dry polymer at low temperatures, and the subscripts represent: hum – humidified, dry – dry, ox – oxide, lt – low temperature, and ht – high temperature.
Figure 8.Schematic representation of the changes in dielectric/cathode interface upon heating/cooling/humidification. Insert – close up of the interfacial regions in the humidified capacitor (region I -as deposited dry PEDOT interface, region II –new interface created upon humidification).
Based on Equations 2–5, the relative changes of the capacitance with temperature can be written as:
The proposed theoretical treatment of the observed changes uses the capacitance values at room temperature (C25C dry and C25C hum) to calculate the values at both high and low temperatures. The primary conclusion from considering Equations 6–9 is equality of the capacitance at 150°C for humidified and dry capacitors. Indeed, the experimental data shown in Figs. 2 and 5 confirm this theoretically derived statement and can serve as a validation of the proposed approach.
The only unknown parameter is ΔCcontr, but it can be estimated from the relative values for two different Ta powders, as shown in Fig. 3. Specifically, the relative change of the C values of the dry samples upon cooling is virtually the same for two different samples, implying that the second component in the Equation 6 is also the same. Consequently, the ratio of ΔCcontr to C25Cdry is the same and can be used to calculate the capacitance change. The above mentioned conclusion is perfectly reasonable when considering that the decrease of the effective area upon contraction should be proportional to the initial covered area at room temperature. In the selected case it is directly proportional.
The relative values calculated based on Equations 6–9 using room temperature data only, together with calculated values based on the actual experimental data at the highest and the lowest temperatures, are presented in Table I and Fig. 9. These results indicate that the theory properly describes the general trend of the data. The numerical values calculated based on our model are very close to the experimental results, and are within ±5%. In our opinion, this theoretical approach based on the contraction/expansion of the cathode explains the observed variation of the capacitance with temperature quite well. It suggests that the contraction/expansion of the cathode with temperature is the underlying mechanism causing the observed changes in the measured capacitance as a function of temperature.
Table I.The values of relative changes of capacitance at the highest and lowest temperatures.
| Sample | ΔC−55C exp, % | ΔC−55C theory, % | ΔC150C exp, % | ΔC150C theory, % | |
|---|---|---|---|---|---|
| 50,000 | dry | −11 | −10 | 26 | 31 |
| humidified | −23 | −19 | 10 | 9 | |
| 12,000 | dry | −10 | −10 | 18 | 21 |
| humidified | −17 | −14 | 11 | 9 | |
| Silane | dry | −6 | −10 | 14 | 18 |
| humidified | −12 | −11 | 5 | 9 | |
Figure 9.Relative changes of the capacitance at the highest and lowest temperatures.
Conclusions
The results presented for Polymer Ta capacitors with slurry PEDOT cathodes show some interesting results with respect to capacitance stability with temperature. Various capacitor structures were investigated, including humidified and dry, in-situ and slurry PEDOT cathodes, coarse and fine tantalum powders, and different dielectric thicknesses. One clear observation was that the capacitance in all cases increased with temperature. The extent and nature of this increase, however, depends on the nature of the slurry cathode and its interface with the dielectric. It is also apparent that capacitors fabricated with coarser powder have less temperature sensitivity, and this effect is primarily due to the better impregnation of the slurry into the tantalum anode.
Furthermore, from Fig. 3 we can see that the inherent variation of the dielectric constant with temperature cannot fully explain the observed results. The capacitance sensitivity to temperature and humidity is rather complicated, where humidified devices show less capacitance variation above room temperature while dry devices show less capacitance variation below room temperature for both coarse and fine powders. The low temperature behavior was shown not to be due to the freezing of water since capacitors exposed to both water and ethanol vapor showed nearly identical C(T) curves, even though the freezing temperature of ethanol is much lower than the temperatures used.
Based on our experimental results, the dominant effect appears to be the coverage of the dielectric with the conductive polymer cathode. With the addition of a Silane coupling agent applied to the dielectric surface, the difference in capacitance between humidified and dry Polymer Tantalum capacitors was reduced and the capacitance dependence on temperature was improved over the entire range of operating temperatures. A model was developed based on these results through consideration of effective surface area changes upon heating or cooling complimented by changes in the dielectric constant of Ta2O5.
The model assumes that the PEDOT layer can shrink upon cooling and expand upon heating, and as a result, the effective surface area contributing to the overall device capacitance decreases or increases accordingly. The PEDOT layer can also expand upon humidification, leaving less room for additional expansion upon heating; and also, it can contract upon drying, leaving less room for additional contraction upon cooling. Experimental and theoretical results were in agreement within ±5%. From these results we conclude that the contraction/expansion of the PEDOT cathode with changes in temperature is the underlying mechanism governing the capacitance stability with temperature of the Polymer Tantalum capacitors. Future research efforts will focus on further improvements in coverage of the Ta2O5 dielectric with slurry PEDOT inside porous tantalum anodes, which will lead to higher capacitance stability with temperature in these capacitors.
Acknowledgments
The authors thank KEMET’s Steve Hussey and Jonathan Paulsen, and Tel-Aviv University’s Dr. Alexander Gladkikh, for their assistance with the fabrication and testing of these capacitors along with helpful discussions.
This article has been published on EPCI website under ECS Journal of Solid State Science and Technology open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY). The article can be viewed and downloaded from the original source here.
References
1.Koch N.,Vollmer A.,Elschner A., Appl. Phys. Lett., 90, 043512 (2007).
2.Ueno K.,Dominey L.,Alwitt R., 211th Meeting of The Electrochemical Society–B1-Electrochemistry of Novel Electrode Materials for Energy Conversion and Storage, May 6–10, 2007.
3.Freeman Y.,Harrell W. R.,Luzinov I.,Holman B.,Lessner P., J. Electrochem. Soc., 156 (6) G65 (2009).
4.Freeman Y.,Alapatt G. F.,Harrell W. R.,Lessner P., J. Electrochem. Soc., 159, (10) A1646 (2012).
5.Young J.,Qiu J.,Hahn R., Proceedings of the 28th Symposium for Passive Electronic Components, March 17–20, 2008.
6.Merker U.,Lovenich W.,Wussow K., Proceedings of the 20th Annual Passive Component Symposium, September 25–28, 2006.
7.Freeman Y.,Alapatt G. F.,Harrell W. R.,Luzinov I.,Lessner P.,Qazi J., ECS J. Solid State Sci. Technol., 2, (11) N197 (2013).
8.Hahn A. R.,Lessner P.,Kinard T.,Melody B., Pat. US 6,334,966 B1 (2002).
9.Qiu Y.,Hahn R.,Brenneman K., U.S. Pat. US 7,563,290 B2 (2009).
10.Merker U.,Lovenich W.,Wussow K., Pat. Application Publication US 2007/0064376 A1.
11.Freeman Y.,Qiu Y.,Hussey S.,Lessner P., U.S. Pat. 8,310,815 B2 (2012).
12.Young L., Anodic Oxide Films, Academic Press, New York, 1961.
13.Abuetwirat I., Dielectric Properties of Thin Tantalum and Niobium Oxide Layers, Doctor Thesis, Brno University of Technology, Brno, 2014.
14.Sakata K.,Aoki Y.,Nishiyama T.,Arai S.,Nagashima S., U.S. Pat. 5,729,429 (1998).
15.van Ooij W. J.,Sabata A., Surf. Interface Anal., 20, (5), 475 (1993).