Research undertaken by Hsu Hao-Lin et. al. from Green Energy Technology Research Center, Kun Shan University, Taiwan propose environmentally friendly and low-cost synthesis route of active composite electrode materials for supercapacitor application comprising of 3D bundle-type carbon nanotubes (BCNTs) with metallic alloys.
They are re-proposing the synthesized BCNTs growth mechanisms in preparing working electrodes using a two stage process with a two-component intermetallic alloy of Ni and Mg (NMA). First, the metals are mixed at a low temperature with a liquid ethylene glycol (EG) medium, followed by the BCNTs being synthesized by catalytic thermal chemical vapor deposition (CVD) which decomposes and drives off the hydrocarbons. In the second processing step, an additional precursor, in the form of molybdenum chloride, is introduced to blend with the NMAs resulting in fast charge transfer between electrode and electrolyte.
The resulting BCNT structures were observed using a field-emission scanning electron microscope (FESEM) and high-resolution transmission electron microscopy (HRTEM) to reveal their structural and surface morphologies, allowing their SSA to be ascertained.
Highlights
- Three-dimensional BCNT composite is the first study of supercapacitor electrode application for publication.
- BCNT electrodes achieve a maximum capacitance of 1,560 F/g
- BCNT electrodes exhibit an energy density of 195 Wh/kg at a power density of 0.21 kW/kg
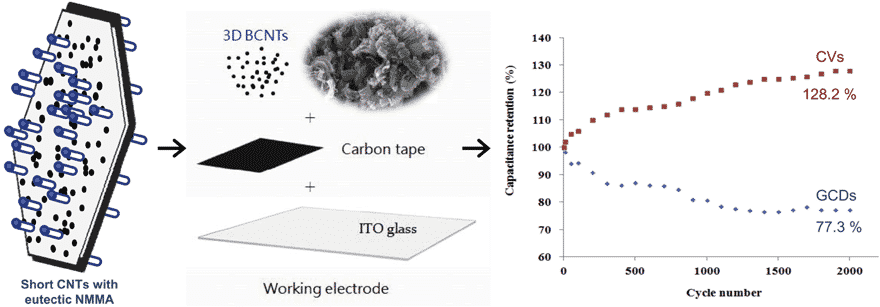
- BCNT electrodes perform specific capacitance retention (128.2%) after 2,000 continuous cyclic voltammetry cycles
Abstract
Bundle-type mutil-walled carbon nanotubes (MWCNTs) composite electrode is the first investigation and publication for the supercapacitor application. According to the thermogravimetric analysis results, as-synthesized BCNTs are considered as the electrode materials for supercapacitors and electrochemical double-layer capacitor in this study. The Brunauer–Emmett–Teller specific surface area of as-prepared bundled carbon nanotubes (BCNTs) is 95.29 m2/g given to a type III isotherm and H3 hysteresis loops.
Slow scanning rates promote and enhance to achieve high Cb because of the superior conductivity of CNT bundles and one side close-layered Ni/Mg/Mo alloy inside the BCNT-based electrode and facile electron diffusivity between electrolyte and electrode. The specific capacitance Cs (1,560 F/g) is nearly equal to the maximum specific capacitance, which the BCNT-based composite electrode can actually be able to charge or fill in. The maximum energy density value is 195 Wh/kg with corresponding power density values of 0.21 kW/kg. Furthermore, the active 3D BCNTs material fabricated electrode enhances to contact the electrolyte directly and decreases the ion diffusion limitation.
Electrochemical impedance spectroscopy spectrum summarized as the low-frequency area controls by mass transfer limitation, and the high-frequency area dominates by charge transfer of kinetic control. After 2,000 consecutive cyclic voltammetry sacnings and galvanostatic charge-discharge cycles at a current density of 1.67 A/g performs, the specific capacitance retentions of 3D BCNTs electrodes achieved 128.2 and 77.3%, respectively. Three-dimensional BCNT composite electrodes exhibit good conductivity and low charge transfer resistance, which is beneficial to fast charge transfer between the BCNTs electrode materials and electrolytes.
Reference
Hsu Hao-Lin et. al. (2021) Three-dimensional bundle-like multiwalled carbon nanotubes composite for supercapacitor electrode application Materials Today Chemistry 22 100569 https://doi.org/10.1016/j.mtchem.2021.100569