Researchers from Czech Advanced Technology and Research Institute (CATRIN), Olomouc demonstrated superior properties of flurographene-derived nitrogen doped graphene (GN3) as efficient supercapacitor or LiS battery electrode, achieving record power and energy densities. The results were published by Power Electronic Devices and Components by Elsevier journal.
Abstract
Fluorographene exhibits a rich chemistry and a wide range of applications in energy storage devices.
This review, which is based on our lab results acquired in the last decade, explores the synthesis, properties, and performance of fluorographene-based materials in supercapacitors and batteries. Fluorographene can be prepared through mechanical or chemical delamination of graphite fluoride, allowing for scalable synthesis and further chemical processing. The chemical versatility of fluorographene enables a wide portfolio of chemical reactions, leading to a new class of graphene derivatives.
Graphene acid, a product of fluorographene chemistry, exhibits excellent specific capacitance, cycling stability, and rate capability. Hybridizing graphene acid with metal-organic frameworks can achieve even higher energy and power densities. Furthermore, nitrogen-doped graphene derived from fluorographene demonstrates remarkable capacitive behavior, making it an efficient electrode material for supercapacitors.
Additionally, fluorographene-based materials, such as graphene acid, graphene-sulfur hybrids, and graphene-based anodes, have exhibited outstanding performance in lithium-ion and lithium-sulfur batteries. The scalable synthesis, high performance, and versatility of fluorographene-derived materials render them attractive for practical energy storage applications. The unique properties and wide range of chemistries offered by fluorographene chemistry open new possibilities for improving advanced energy storage devices.
Fluorographene Derivatives
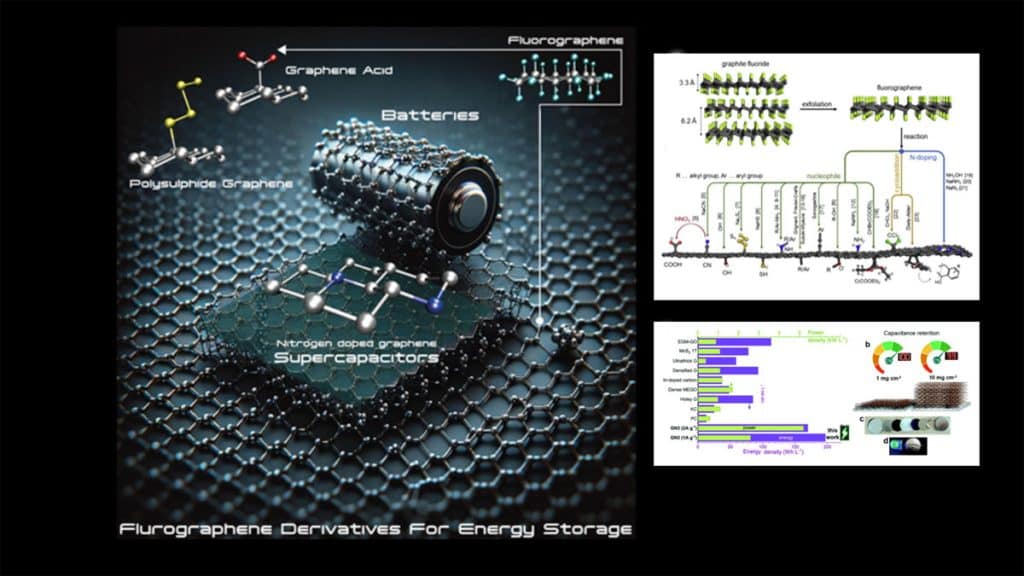
Fluorographene, which was discovered in 2010, can be prepared i) up-down by mechanical or chemical delamination of graphite fluoride and ii) bottom-up by fluorination of graphene. Particularly the chemical exfoliation is suitable for upscaling fluorographene synthesis and further chemical processing. As a perfluorinated hydrocarbon labeled as a two-dimensional Teflon counterpart, it was considered unreactive due to the strength of the carbon-fluorine bond, which is regarded as one of the strongest single chemical bonds.
The C-F bonds on tertiary carbon atoms, which make the backbone of fluorographene, are considered an Achilles heel of fluorocarbons. In addition, real fluorographene samples contain radical defects, which are strong electrophiles and trigger a rich chemistry of fluorographene, leading to a broad portfolio of surface functionalized graphenes.
The utilization of fluorographene chemistry as a tailored way to prepare novel graphene-based materials aligns with Regulation of Hazardous Substances (RoHS) directives. The end products resulting from fluorographene chemistry, comprised of naturally abundant elements, emerge as promising contenders due to their outstanding performance and as a significant stride towards a complete dedication to sustainable practices. It is worth noting, however, that a comprehensive Life Cycle Assessment (LCA) of fluorographene chemistry, which thoroughly evaluates the environmental impacts of products and processes across their entire life cycle, has not been published to date.
Derivatized fluorographene as supercapacitor electrode material
Higher efficiency of derivatized fluorographene in supercapacitors was demonstrated on partially isothermally reduced fluorographene at 450°C, which was prepared in a reducing atmosphere of hydrogen. The final composition, i.e., carbon sp2/sp3 ratio, of the partially reduced fluorographene was fine-tuned by thermal treatment time.
Nitrogen-doped graphenes obtained by reducing fluorographene using nitrogen-containing compounds represent fascinating class of materials highly suited for supercapacitor electrodes. Among them, GN3 (prepared by the reaction of fluorographene with sodium azide) stands out as a nitrogen-doped graphene with exceptionally high levels of nitrogen (∼16 %) and containing diamond-like bonds. Compared with graphite, it has a higher density of 2.8 g/mL. This unique material exhibits unprecedented capacitive behavior. When utilized as active material in a symmetric capacitor, paired with an ionic liquid electrolyte (1-ethyl-3-methylimidazolium tetrafluoroborate, EMIM-BF4, and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE) in a 9 : 1 ratio), it displays remarkable volumetric energy density (200 Wh/L) and power density (52 kW/L) while maintaining excellent cycling stability. Since such values are record among the high-performance supercapacitor materials, commercialization of the nitrogen-doped graphene (labeled as SC-GN3) is under development with the support of the EIC Transition project (trans2Dchem.com) funded by the European Union. The qualification of the initial prototypes, featuring both wound (cylindrical) and pouch geometries, is scheduled for 2024.
Fluorographene-derived materials as Li battery electrodes
The effectiveness of materials derived from fluorographene has also been demonstrated in lithium batteries as efficient electrode materials. In the case of graphene acid, its carboxylic groups can reversibly bind lithium ions, making it a promising high-energy anode.
Experiments have revealed that the graphene acid anode exhibits exceptional charge transport, Li intercalation properties, and redox activity at the single-layer level while maintaining the electrode stability. This behavior surpasses all previously reported organic anodes, incorporating commercial graphene and nanoplatelets of graphene. With a practical capacity of 800 mAh/g (0.05 A/g) and a rate capability of 174 mAh/g (2.0 A/g), the graphene acid anodes demonstrate their true potential in advanced lithium-ion batteries.
The chemistry of fluorographene also enables the covalent conjugation of graphene with polysulfide chains, resulting in the development of polysulfide covalently-interlinked graphene (GPS). This remarkable material exhibits exceptional potential as a Li-sulfur battery cathode nanomaterial due to its outstanding characteristics.
The electrochemical profiles during galvanostatic charge/discharge were maintained over 50 cycles at a specific current of 0.1 C, highlighting the high electrochemical reversibility of the GPS cathode. The GPS material exhibited exceptional stability at high and low specific currents, retaining initial capacities above 470 mAh/g even after 500 cycles at 1 C current. Moreover, the GPS cathode achieved capacities of 485 mAh/g at 1 C and 290 mAh/g at 2 C. Compared to noncovalent graphene-sulfur (GS) derivatives, the GPS cathode outperformed them, exhibiting superior stability compared to other sulfur-based materials investigated for lithium-sulfur batteries. The stability of GPS over GS is further evidenced by the absence of elemental sulfur in the separator after 250 cycles. In contrast, sulfur was observed in the GS sample after only 45 cycles. The lasagna-like structure of GPS prevented the sulfur shuttling effect, which typically stands behind a low cyclability of Li-S batteries (LSBs).
Summary Highlights
- •Versatile fluorographene chemistry enables scalable synthesis of graphene derivatives for diverse energy storage applications.
- •Graphene acid, a supercapacitor electrode, exhibits high capacitance, enhanced through covalent modifications.
- •Graphene acid anode increases the storage capacity of Li-ion batteries.
- •Nitrogen-doped graphene proves efficient as a supercapacitor electrode, achieving record power and energy densities.
- •Sulfide chains interlinking graphene sheets enhance cycling stability in Li-sulfur batteries, preventing the shuttling effect.
Conclusions and Perspectives
Fluorographene chemistry offers an industrially scalable process that leads to a new class of densely homogeneous and surface-functionalized graphenes.
These materials exhibit immense versatility for a broad range of energy storage applications, containing, for instance, supercapacitors and batteries. Among supercapacitor electrodes, GN3 stands out with its exceptional energy and power densities, making it an up-and-coming candidate for the transition of graphene supercapacitor devices into real-world applications soon. On the other hand, graphene acid, the most versatile among fluorographene-derived materials, exhibits excellent capacitive behavior in a simple system of aqueous sulfuric acid electrolytes, offering a cost-effective and eco-friendly alternative.
The capacitive abilities of graphene acid can be further enhanced through hybridization with metal-organic frameworks (MOFs), opening up new routes for improved energy storage performance. Additionally, graphene acid exhibits remarkable potential as a high-energy content anode in organic lithium batteries, thanks to its reversible reaction with lithium ions, leading to significant performance enhancements.
Lastly, owing to its interlinked graphene superstructure character, GPS material may serve as an unprecedent cathode material in Li-S batteries, overcoming the sulfur shuttling effect and retaining high capacity over time. Overall, the tested fluorographene-derived materials have proven to be highly efficient electrode materials for applications related to energy storage.
Given the elegance of fluorographene chemistry, we anticipate the emergence of more fluorographene-derived materials, expanding the possibilities in energy storage devices and further pushing their efficiency limits.
Acknowledgments
The work was supported by ERC (2Dchem ID:683024), ERC PoC (UP2Dchem ID:899245 and FunGraB ID:101069293), and EIC Transition Trans2Dchem (ID:101057616) grants.
We also acknowledge support from the ERDF/ESF project TECHSCALE (No. CZ.02.01.01/00/22_008/0004587) and the financial support of the European Union under the REFRESH – Research Excellence For REgion Sustainability and High-tech Industries project number CZ.10.03.01/00/22_003/0000048 via the Operational Programme Just Transition.
This review also relates to the presentation given by Michal Otyepka at the 4th PCNS Passive Components Networking Symposium conference held from September 11th to 14th, 2023, in Sønderborg, Denmark.
Journal Reference
Vítězslav Hrubý, Veronika Šedajová, Petr Jakubec, Aristeidis Bakandritsos, Radek Zbořil, Michal Otyepka,
Unleashing the Power: Superior Properties of Fluorographene-Derived Materials for Energy Storage Applications,
Power Electronic Devices and Components, 2024, 100058, ISSN 2772-3704,
https://doi.org/10.1016/j.pedc.2024.100058.