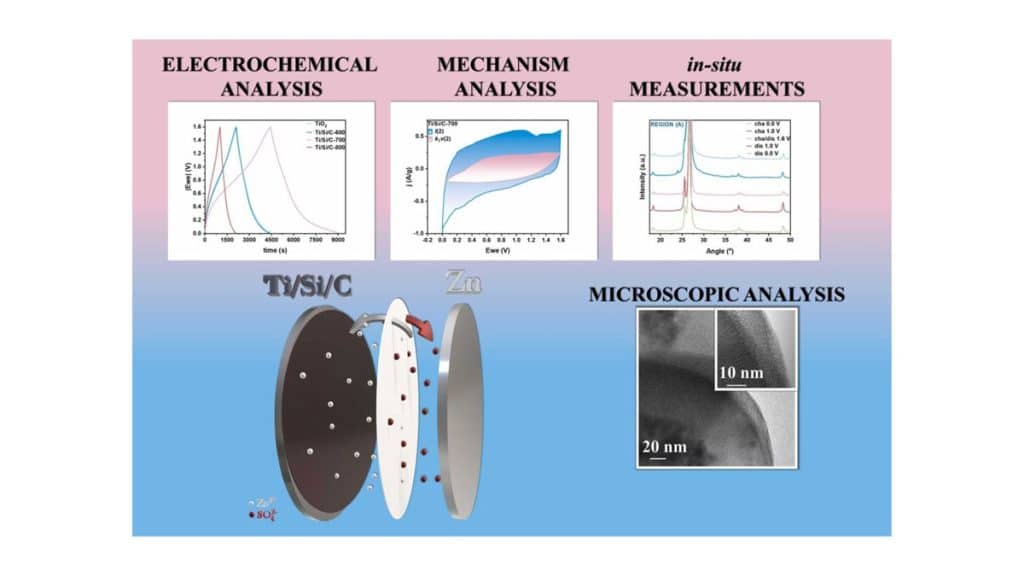
Researchers from West Pomeranian University of Technology in Szczecin, Poland published paper “Core/shell structured Ti/Si/C composite for high-performance zinc-ion hybrid supercapacitor” in Journal of Alloys and Compounds.
In this study, novel nanocomposites with a core/shell structure based on titania/silica/carbon nanoparticles (Ti/Si/C) were fabricated using a solvothermal method and subsequent carbonization. The resulting material showed superior activity in a hybrid energy storage system using a zinc-ion supercapacitor as a model device. The TiO2 nanoparticles served as the core of the composite, providing a high surface area for electrochemical reactions, enhancing electrical conductivity.
Abstract
Zinc-ion capacitors (ZnIC) have focused great attention due to high intrinsic safety and their advantages in terms of environmental protection and price. However, the increase in energy and power densities still remains a challenge.
Therefore, this study aims to design and fabricate novel low-cost and environmentally-friendly nanocomposite based on titania/silica/carbon used as cathode in ZnIC. The fabrication procedure involves the functionalization of TiO2 nanoparticles with APTES and glucose, followed by a hydrothermal reaction and carbonization at 600, 700, and 800 °C (Ti/Si/C-Tx, where Tx is the temperature of carbonization) to result in carbon-coated titania/silica with a core/shell architecture. In-situ Raman and X-Ray Diffractometry (XRD) analyses were conducted to elucidate the electrochemical behaviour of the material during charge-discharge cycles.
The nanocomposites were electrochemically tested using cyclic voltammetry (CV), galvanostatic cycling with potential limitation (GCPL), and potentio electrochemical impedance spectroscopy (PEIS). The designed nanocomposite showed improved performance in terms of specific capacity, power, and energy densities with values of 295 F/g, 160 W/kg, and 210 Wh/kg, respectively.
Ti/Si/C-700 also demonstrated a high stability retention of 85% after 10′000 cycles, indicating its potential for use in energy storage devices. Despite these promising results, further research is required to improve capacity retention at increased current densities. The key implication of this study is the potential for the development of low-cost and high-performance energy storage devices for various applications, contributing to the growth of renewable energy sources.
Introduction
Currently, one of the most promising energy storage technology, which can fill the gap between supercapacitors and batteries, is based on an electrochemical device called Zinc-ion supercapacitors (ZnIC). The mechanism includes simultaneous adsorption-desorption of ions on one electrode and zinc-ion covering/fracturing on the second Zn electrode.
Here, the storage mechanism is based on porous carbon storage via the double-layer capacitance, due to the Faradaic behaviour of the Zn electrode that exhibits flat charge/discharge plateaus. Theoretically, a porous carbon with a specific surface area (SSA) in the range between 1000 and 3000 m2/g can reach a capacitance of 300–500 F/g. In practice, they reach only 100–250 F/g.
Nonetheless, there are still challenges with ZnIC, and their primary drawback is the specific capacity and kinetic imbalance between the electrodes due to the different energy storage mechanisms of capacitor-type and battery-type electrodes. To overcome this obstacle we designed a fabrication of a new carbon/titania/silica-based composite, which served as a promising cathode in ZnIC.
Our approach induces an increase in the electrochemically available active surface areas (through porous carbon) and induces pseudocapacitance due to the presence of SiO2 and TiO2. To the best of our knowledge, this particular approach has never been studied before but the potential of silica and titania-based compounds in supercapacitors has been addressed.
Here, we address the issues using a facile fabrication route of a three-component molecular hybrid composed of a core/shell structure based on TiO2 nanoparticles coated by silica and graphitic shell (Ti/Si/C). The designed material was tested in ZnIC using carbon-based EDLC as positive electrodes and zinc foil as negative electrodes in ZnSO4·7 H2O electrolyte.
The encapsulation of TiO2/SiO2 in carbon spheres provided room to gather the charge near the TiO2 particles (surface storage), and modification with Si species improved device capacity. The approach allowed for overcoming the limitation of low performance observed in TiO2-based electrodes for energy storage. The method involves the synthesis of ultrasmall particles from a semiconductor core.
These particles are prevented from agglomerating through silica and carbon coatings. This design induces more active sites during the energy storage process. Furthermore, this strategy resulted in a composite fabrication with remarkable electrochemical properties, such as a high capacity of 295 F/g (at 0.1 A/g). This is one of the highest in the current state of the art.
Furthermore, the analysis of EDLC and pseudocapacitance contributions revealed a cooperative effect between diffusion-controlled and surface capacitance-controlled mechanisms, with surface capacitance being the dominant mechanism. In addition, the electrochemical behaviour of the material was further elucidated through in-situ Raman and XRD analyses during the charge-discharge process.
The results shed light on the underlying electrochemical behaviour of the material behind its improved performance. Thus, the material can deliver energy at a huge speed, with the possibility to repeat the cycles of charging and delivering energy for at least thousands of repetitions. The facile synthesis route, outstanding electrochemical performance, and in-situ analysis make this work highly significant for the development of high-performance energy storage materials.
Read more in reference: https://doi.org/10.1016/j.jallcom.2023.171259