KEMET, part of YAGEO Group, offers miniature EDLC supercapacitor that uses a unique aqueous electrolyte solution suitable for high reliability automotive applications.
KEMET is the only producer of supercapacitors using aqueous electrolyte solutions in the world.
Aqueous electrolytes are highly conductive, low environmental impact, non-toxic, and non-flammable, yielding strong performance and safety credentials. They also typically have greater resistance to moisture absorption than organic compounds, resulting in a longer lifetime with better stability.
The electrolyte of dilute sulfuric acid maintains high durability even under high temperature and high humidity conditions. A proprietary separator membrane has been developed from a high heat-resistant material whose pore diameter does not close even after extended use to improve the robustness at high temperatures.
Small-cell super capacitors from KEMET feature a high-strength vulcanized rubber bond to ensure against liquid electrolyte leakage. The cross-section shown in figure 1 explains how these supercapacitors are constructed, including the aqueous electrolyte, rubberized electrodes, and separator membrane.
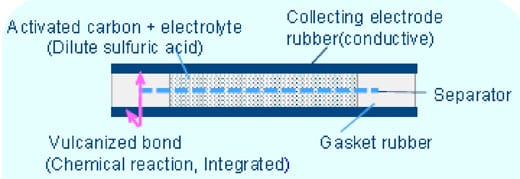
These features make KEMET supercapacitors an ideal choice for high reliability, harsh environments, including automotive applications. KEMET has released two supercapacitors that are qualified to an automotive testing protocol. These capacitors are manufactured in an ISO TS 16949 certified plant and are subjected to PPAP/PSW and change control.
Supercapacitors For Automotive
Modern advances in carbon-based materials enable porous electrodes to have a large surface area resulting in a high capacitance density and small physical form factor. Such high volumetric efficiency makes them an ideal candidate for replacing small secondary coin cell batteries. The ionic processes used to store energy in supercapacitors are also relatively fast. The device can fully charge within a few seconds, whereas a typical battery cell can take from ten minutes to several hours to fully charge. Moreover, there is no theoretical limit to a supercapacitor life cycle, whereas a lithium-ion secondary cell has a finite lifetime of about 500 cycles.
For all device types, the electrolyte properties determine the overall super capacitor terminal voltage. The voltage, when fully charged, is usually less than 3V. A conventional approach to constructing supercapacitors is comparable to that of a coin cell: lower and upper metal cases are joined by swaging to enclose the carbon electrodes and organic electrolyte.
With capabilities including high life cycle and fast charge and discharge times, small-cell supercapacitors can oust coin-type batteries from backup power duties in equipment ranging from IoT devices, smart meters, or medical devices to automotive electronics and industrial computing.
In automotive applications, the need for system backup during a power loss is becoming increasingly critical as connectivity and autonomous driving technology gain traction in modern vehicles. The automotive environment, however, is extraordinarily harsh with high temperature, humidity, and vibration. Traditional electrolytic capacitors and batteries will dry out over time under high-temperature conditions, resulting in increased series resistance and degradation of capacitance.
Specialized supercapacitors can be used in these situations to set design engineers free from the restrictions imposed by finite battery lifetimes. In addition, the supercapacitor’s benign open-circuit failure mode contrasts with typical short-circuit battery failures that may result in out gassing or ignition. As such, supercapacitors are the most cost-effective alternative to small backup batteries and can store enough energy to provide backup for durations ranging from a few seconds to several days, depending on the type of load and current demand.
Figure 2. presents KEMET supercapacitor FMD0H334ZF, which offers 0.33F and 5.5V. This high-capacity supercapacitor is built with a resin-molded package that enables tape-and-reel packaging and is compatible with automated mounting. It is the world’s first supercapacitor that is rated for 1,000 hours in a high temperature and high humidity environment at 85°C-85% and is also qualified to an automotive testing protocol for an operating temperature range of -40°C to 85°C.
Figure 3. depicts another product, the FU0H105ZF supercapacitor, that offers 1F at 5.5V with the outer packaging built from a sealed metal can. This product is qualified to an automotive testing protocol and is a long-life device with operation exceeding 4,000 hours at 85°C. This harsh environment lifetime is equivalent to 10 years of life when referring to the mission profile near the dashboard of an automobile.
Conclusion
Supercapacitors offer a high-performance alternative to batteries in many backup-power applications, delivering far greater cycle life and avoiding any need for designers to worry about battery replacement or recharging.
The latest supercapacitors using KEMET aqueous electrolyte are cutting-edge energy storage devices featuring high voltage, long life, and environmental resistance required by the automotive market. KEMET new supercapacitors are ideal for use in automotive, medical, aerospace, industry, and other areas as required for high-reliability performance.