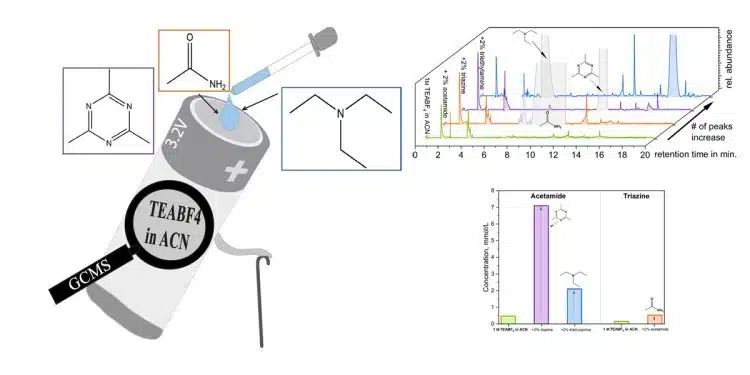
An ongoing challenge in the field of supercapacitors revolves around comprehending their failure mechanisms. Researchers from Friedrich-Schiller-University Jena, Germany proposes the use of post-mortem Gas Chromatography-Mass Spectrometry Analysis (GS-MS) to gain a deeper understanding of the importance of individual decomposition products on the overall decomposition of the electrolytic solution of supercapacitors. This analytical technique can become a very powerful tool for interpreting and understanding the degradation processes occurring in supercapacitor electrolytes.
Abstract
The aging processes occurring at the electrode/electrolyte interphase of these devices are complex. Thus far, much attention has been directed toward examining the aging of electrodes, while fewer studies have been dedicated to the electrolyte’s aging. This study aims to address this point and to gain a deeper understanding of the importance of individual decomposition products on the overall decomposition of the electrolytic solution of electric double-layer capacitors.
Therefore, the decomposition of the state-of-the-art electrolyte 1 m solution of tetraethylammonium-tetrafluoroborate in acetonitrile and that of the same electrolyte doped with compounds known to form during its aging, such as acetamide, 2,4,6-trimethyl-1,3,5-triazine, and triethylamine has been investigated post-mortem. The results of this study show that GC-MS is a useful technique to interpret and understand the degradation processes taking place in the electrolytes of supercapacitors.
Introduction
Electric double-layer capacitors (EDLCs) are considered among the most important energy storage devices utilized in our daily lives. They display high specific power, the advantages of fast charge and discharge times, and a long operational lifetime. Due to these benefits, they are implemented in a large number of applications, e. g. transportation, grid stability, memory backup, or wind turbines. The storage processes occurring in EDLCs are of physical nature. The formation/depletion of an electric double-layer at the electrode/electrolyte interphase determines the features of these high-power devices, and thus affect the aging.
The aging of EDLCs, which typically leads to loss of capacitance and increase in resistance is a complex process that involves various failure mechanisms, each playing a crucial role. These mechanisms involve structural and chemical changes, material dissolution, and corrosion of metal current collectors during cell operation. All of them can impact performance under varying operating conditions like temperature, humidity, and voltage, with increased temperatures potentially inducing undesired chemical reactions and low temperatures causing electrolyte freezing and mechanical stress. In the past most effort has been directed towards examining and understanding the aging occurring on the electrodes of EDLCs.
Less attention has been dedicated to the processes occurring with the electrolyte. It has been shown that the degradation of the electrolyte, e. g. during high voltage test, might lead to the generation of gases and side products which, in turn, lead to increased pressure and pore blocking. The evaluation of formed gases and electrode decomposition products has been studied rather intensely. In contrast, several aspects related to the electrolyte’s aging are still poorly understood. For example, it is not fully clear how the different electrolyte decomposition processes affect the performance of EDLC’s.
Gas chromatography-mass spectrometry (GC-MS) is well-suited for examining trace amounts of dissolved analytes in a solvent, making it an ideal technique for investigating formed degradation products within the electrolyte of an electrochemical storage device.
It would be extremely helpful to identify strategies to prevent, or at least alleviate, the degradation of EDLCs. Therefore, it must be understood to which extend each individual decomposition product contributes to the overall aging process of EDLCs. In this work a strategy is proposed to understand the impact of known decomposition compounds, namely acetamide, 2,4,6-trimethyl-1,3,5-triazine (triazine) and triethylamine, of the aging of EDLCs containing 1 m TEABF4 in ACN as electrolyte.
Results and Discussion
Initially, the transport properties of the investigated electrolytes have been considered. The viscosity and the conductivity of 1 m TEABF4 in ACN with the addition of 2 wt.% acetamide, 2 wt.% 2,4,6- trimethyl- 1,3,5- triazine (triazine), and 2 wt.% triethylamine are compared (Figure S1), with the reported values given for 20 °C. The pristine electrolyte displays viscosity and conductivity values of 0.78 mPa s and 66.8 mS cm−1, respectively and correspond with those reported in literature. The addition of dopants to the electrolyte results in a less favorable condition for transport properties. Therefore, the least favorable values are given by the addition of 2 wt.% triazine, which displays the highest viscosity and the lowest conductivity (0.93 mPa s and 50.2 mS cm−1, respectively).
The fastest degradation happened with the addition of 2 wt.% of the triethylamine, where the charge-discharge profile is barely visible after 2 h. Apparently, even pre-cycling at 1.6 V cell voltage resulted in substantial degradation, evident from the sloping charge-discharge curves. It can be concluded that the presence of this decomposition compound contributes the most to the aging and, therefore, is expected to be the most detrimental for the cell stability.
Considering that literature often claims the aging of acetonitrile to be more deteriorating than the salt, the clear rise in 3-aminocrotononitrile after adding triethylamine implies that the potential impact of salt degradation could be a factor worth exploring. For the decomposition of the salt, triethylamine only forms at the negative electrode via the Hofman elimination.
The GC-MS analytical method offers significant advantages, allowing for both the qualitative identification of unknown compounds and the quantitative determination of compounds.
Conclusions
In this study, we showed that GC-MS can represent a helpful tool to understand the aging of EDLCs. Utilizing a specifically designed cell for post-mortem GC-MS measurements, we investigated the aging of EDLCs containing 1 m TEABF4 in ACN, as well as that of this electrolyte doped with 2 wt.% acetamide, 2 wt.% 2,4,6-trimethyl-1,3,5-triazine and 2 wt.% triethylamine.
We showed that the most relevant peaks found in the investigated cells, besides the doped compounds, were decomposition compounds of diacetamide, N-ethylacetamide and 3-aminocrotononitrile. Interestingly, the addition of 2 wt.% triethylamine accelerated the formation of 3-aminocrotononitrile, leading to the strongest deterioration of the cell. To make possible the realization of a quantitative comparison between the TICs, a 7-point calibration was implemented. The results were consistent with the qualitative findings, indicated by the occurrence of a faster degradation due to the presence of triethylamine. All values depicted were magnitudes higher than the undoped electrolyte.
The results of this investigation are indicating that post-mortem GC-MS measurements can supply important information, from the qualitative and quantitative point of view, about the degradation processes taking place in EDLCs. In the future, it will be important to extend this type of investigation on systems containing alternative electrolytes.
Furthermore, it will be helpful to combine this technique with methods such as OEMS/DEMS to gain knowledge about the gas-phase/gas formation in EDLCs, and the electrode surface through Raman or XPS to better understand the electrode-electrolyte interplay and its impact on the aging of EDLCs.
Finally, this methodology can also be implemented to other systems such as battery or hybrid materials to extend the knowledge of their aging processes.
Reference Complete Article Link:
“Post-Mortem Gas Chromatography-Mass Spectrometry Analysis of Aging Processes in Acetonitrile-based Supercapacitors”,
Rebecka Kost, Fabian A. Kreth, Prof. Dr. Andrea Balducci; Friedrich-Schiller-University Jena, Institute for Technical Chemistry and Environmental Chemistry (ITUC) and, Center for Energy and Environmental Chemistry Jena (CEEC Jena), Germany
ChemElectroChem Journal; https://doi.org/10.1002/celc.202300823