Researchers at MIT say they have created a new organic based material suitable for high energy storage with fast charge transport.
MIT researchers in the journal Joule, report new material suitable for energy storage that combines high charge capacity of batteries with “long cycling life of capacitors.”
Lithium-ion cells (LIC), they explain, are the leading electrochemical energy storage devices in use today. They boast high charge capacity but fall short when it comes to recharging times.
As an alternative, designs that incorporated plentiful, inexpensive organic materials were proposed. But they were found to suffer from diminished electrical conductivity. Other approaches that utilized modular designs that combined LICs and supercapacitors within specific devices proved overly complex and costly.
Those problems, according to MIT graduate student Tianyang Chen, author of the Joule report, “create a strong incentive to develop electrode materials that combine the high charge capacity of LICs with the fast charging and long cycling life of capacitors.”
Chen and his team came up with a combination of organic materials that can be used in battery cathodes, where lithium is stored in discharged batteries.
Attempts by others to use organic materials failed because they dissolved in battery electrolytes.
“Although strategies such as polymerization and compositing organic molecules with insoluble admixers can limit electrode dissolution,” Chen said, “designing organic materials that are themselves insoluble but still allow swift charge transport and storage is difficult.”
But the materials created in MIT laboratories remain stable, Chen said.
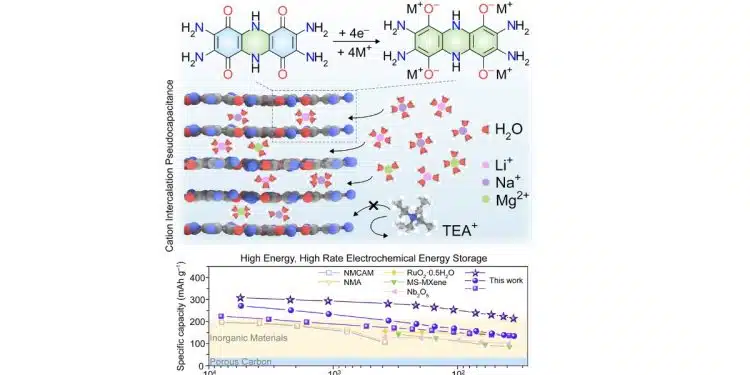
“We report bis-tetraaminobenzoquinone and its polymeric analog poly(bis-tetraaminobenzoquinone) as pseudocapacitive organic materials” for electrical energy storage devices, he said. They “exhibit high charge storage capacities at high charge-discharge rates.”
Abstract
Designing materials for electrochemical energy storage with short charging times and high charge capacities is a longstanding challenge. The fundamental difficulty lies in incorporating a high density of redox couples into a stable material that can efficiently conduct both ions and electrons. We report all-organic, fused aromatic materials that store up to 310 mAh g−1 and charge in as little as 33 s. This performance stems from abundant quinone/imine functionalities that decorate an extended aromatic backbone, act as redox-active sites, engage in hydrogen bonding, and enable a delocalized high-rate energy storage with stability upon cycling. The extended conjugation and hydrogen-bonding-assisted bulk charge storage contrast with the surface-confined or hydration-dependent behavior of traditional inorganic electrodes.
Highlights
- Fused aromatic molecules with abundant heteroatoms are developed for energy storage
- Extended conjugation and hydrogen bonding enable rapid conduction of charges
- pH-dependent pseudocapacitive intercalation charge storage is realized
- A rare combination of high-capacity, high-rate charge storage is achieved
Reference: Tianyang Chen et al, High-rate, high-capacity electrochemical energy storage in hydrogen-bonded fused aromatics, Joule (2023). DOI: 10.1016/j.joule.2023.03.011