source: University of Amsterdam news
Students at the University of Amsterdam’s Research Priority Area Sustainable Chemistry have tripled the specific capacitance of nitrogen-doped carbons: new materials with potential applications in fast energy storage (e.g. for regenerative breaking or fast charging of cellphones). Their experiments required new pieces of equipment which were designed and produced in the lab using 3D printing.
The Amsterdam students investigated the factors that govern different modes of supercapacitance in the new supercapacitor material that was invented at the University of Amsterdam (UvA). They were able to tune the materials’ surface structure and functional groups in order to maximise fast faradaic reactions at the surface, storing energy in transient chemical bonds. Their results have recently been published by the high-impact international journal ChemSusChem.
Fast charge/discharge cycles
The transition to sustainable energy sources requires efficient energy storage solutions. Each renewable energy source (be it wind, solar, geothermal) sets its own requirements for energy density, power density, life-time, cost, and size. Supercapacitors (also called electrochemical capacitors or ultra-capacitors) are important power sources for applications requiring fast charge/discharge cycles.
Supercapacitors operate via two mechanisms. The first is electric double layer capacitance (EDLC), in which charging the electrode leads to adsorption and desorption of electrolyte counter-ions at its surface. This sorption is fast and reversible, determining the high power density of the device and its longevity. The second mechanism is pseudo-capacitance (PC), wherein fast faradaic reactions occur at the surface, storing charge in chemical bonds and boosting the energy density. These redox reactions are fast enough so that diffusion limitations are small and power density remains high.
Recently, a new type of supercapacitor material made from hierarchically porous nitrogen-doped carbon was invented by Dr David Eisenberg and Prof. Gadi Rothenberg of the Van ‘t Hoff Institute for Molecular Sciences.
Building on that invention, sustainable chemistry students Jasper Biemolt and Ilse Denekamp set out to investigate in their MSc project the factors that govern energy storage at the surfaces of these materials via EDLC and PC. They found that by tuning the synthesis conditions they could tailor the number and type of nitrogen functionalities at the surface, thereby enhancing the capacitance nearly threefold.
3D printed ‘minion’
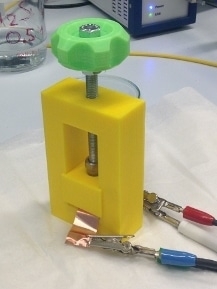
The measurements required a dedicated setup, made from isolating materials to high specifications of mechanical pressure and structural constraints. PhD student Thierry Slot designed the device and printed it on a 3D printer using high-density polystyrene. The device (named “The Minion” for its yellow and green colors) enabled measurement of the capacitance of the new materials.The Amsterdam team included the CAD files to enable researchers who wish to repeat the experiments to 3D-print their own equipment. Rothenberg envisions that more and more researchers will include such files as supporting information with scientific papers:
“We are just starting to realise the potential of 3D printing for the design and printing of tailored-to-purpose lab equipment. As 3D printing becomes more accessible and more types of materials can be printed, designing of equipment for specific experiments will also become easier, and by publishing the CAD files researchers across the globe will be able to print the same equipment in their own labs”.

The different types of nitrogen functionalities on nitrogen-doped carbons and their corresponding pseudo-capacitive reactions. Image: HIMS.
Publication
J. Biemolt, I.M. Denekamp, T.K. Slot, G. Rothenberg and D. Eisenberg: Boosting the supercapacitance of nitrogen-doped carbon by tuning surface functionalities. ChemSusChem, 2017, published online 6 June 2017, DOI: 10.1002/cssc.201700902
featured image: The 3D printed “Minion” device enables measurement of the capacitance of the new materials. Photo: HIMS.