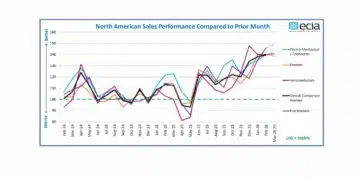
source: AVX news
With more than 2,500 square feet of cleanrooms & support space for device fabrication, biological analysis, & electrical testing, the new facility will further extend AVX’s robust portfolio of components for Class I, II, & III medical devices.
FOUNTAIN INN, S.C. (April 18, 2017) – AVX Corporation, a leading manufacturer and supplier of passive components and interconnect solutions, has added a new medical device development facility to its corporate headquarters in Fountain Inn, S.C. With more than 2,500 square feet of cleanrooms and support space for device fabrication, biological analysis, and electrical testing equipment, the new medical device development lab will accommodate the efforts of eight to ten researchers dedicated to developing cutting-edge medical components. These devices will further extend AVX’s robust portfolio of passive and interconnect solutions especially designed to satisfy the stringent electrical and mechanical performance demands of Class I, II, and III medical devices.
“Backed by more than 20 years’ experience providing advanced passive component and interconnect solutions to the medical industry, our new medical device development facility represents an investment of more than $2.1 million and demonstrates our continued commitment to developing state-of-the-art component solutions for life-saving and life-enhancing technologies,” said John Lawing, CTO and vice president of R&D, AVX. “Our medical design engineers have an innovative approach to problem solving and an in-depth understanding of medical device design requirements, and our quality systems lead the industry and support customer-specific change control, documentation, specification, and testing procedures.”
AVX offers a wide range of advanced tantalum and ceramic capacitors, thin film products, connectors, EMI filters and feedthroughs, SAW filters, and timing devices for medical applications spanning Class III implantable and life-support devices, including: pacemakers, implantable cardioverter-defibrillators (ICDs), and neuromodulation devices, to Class I and II devices, including: cochlear implants, insulin pumps, drug pumps, hearing aids, heart monitors, vascular assist devices, MRI and X-ray machines, external defibrillators, and other monitoring and diagnostic devices.